Procedure expands options for women with uterine factor infertility
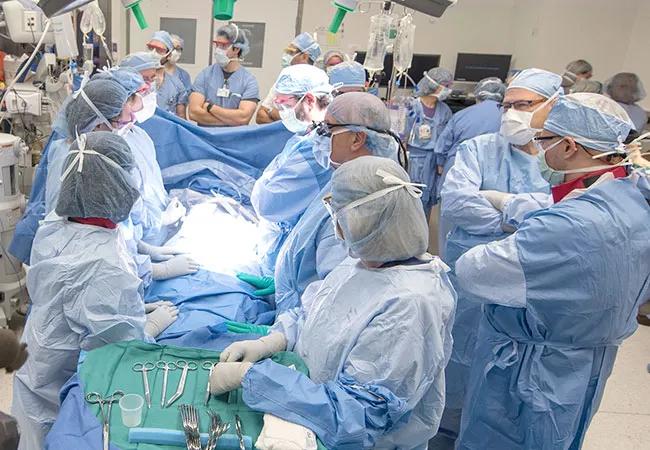
In a first for North America, a Cleveland Clinic patient has given birth after receiving a transplanted uterus from a deceased donor.
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
The transplant was performed in late 2017. In late 2018, the mother, who is in her mid-30s, became pregnant through in vitro fertilization. The delivery via Caesarean section took place in June. The mother and her daughter are doing well.
The birth is the culmination of years of research and preparation by Cleveland Clinic’s multidisciplinary uterine transplant team, which includes specialists in transplant surgery, obstetrics and gynecology, fertility, neonatology, bioethics, psychiatry, nursing, anesthesiology, infectious disease, interventional radiology, patient advocacy and social work.
Although the transplant is still experimental, its success helps validate a new therapeutic option for women of reproductive age with absolute uterine factor infertility (AUFI) when a living donor is unavailable.

“Through our research, we are working very hard to make uterine transplantation a reality for women who choose this approach,” says transplant surgeon Andreas Tzakis, MD, PhD, who has spearheaded Cleveland Clinic’s efforts to develop and advance the procedure. “This case confirms that the birth of a healthy baby is perfectly feasible after a uterine transplant from a deceased donor.”
“The teamwork it took to make this happen for our patient was remarkable. I am so proud,” says Cleveland Clinic obstetrician/gynecologist Tommaso Falcone, MD, former Chair of the Ob/Gyn & Women’s Health Institute. “This clinical trial reflects the Cleveland Clinic tradition of innovation in clinical medicine.”
“We couldn’t have asked for a better outcome,” says Cleveland Clinic maternal-fetal medicine specialist Uma Perni, MD, who managed the patient’s prenatal care and delivery. “The field of uterus transplantation is rapidly evolving, and it is exciting to see what the options may be for women in the future.”
An estimated 1 in 500 women of childbearing age worldwide are affected by AUFI, meaning the absence of a uterus or the presence of a nonfunctional one. Until recently the condition had been considered untreatable, with adoption or surrogacy the only options for parenthood.
Swedish researchers at the University of Gothenburg led by Mats Brännström, MD, PhD, pioneered the uterine transplant concept during the last two decades. Dr. Tzakis, who had been conducting his own uterine transplant research in animal models at the University of Miami, prior to joining Cleveland Clinic, was invited to collaborate with the Swedish team, jointly conducting research and observing the program’s first nine human transplants.
Dr. Falcone, a researcher in the field of infertility and reproductive surgery, partnered with Dr. Tzakis to develop Cleveland Clinic’s uterine transplant program and guide it through the lengthy institutional review and approval process. Dr. Falcone also spent time with the Swedish program to observe surgeries and meet with patients.
Since 2014, there have been more than a dozen live births worldwide resulting from living-donor uterine transplants, including nine in Sweden and two in the United States, at Baylor University Medical Center, in 2017 and 2018.
Deceased-donor transplantation has the potential to increase overall organ supply and patient access to the procedure, while avoiding exposing a living donor to prolonged surgery and extensive pelvic dissection. “There are pros and cons for both the living- and deceased-donor approaches,” Dr. Tzakis says. The surgery to obtain the donor uterus “is quite involved and we were concerned about complications” in a living patient. “We decided to use deceased donors.”
The world’s first birth after a successful deceased-donor uterine transplant occurred in September 2016 and was reported in December 2018 by a surgical team at the University of São Paulo, Brazil.
Using an organ from a deceased donor, Cleveland Clinic’s team performed the nation’s first uterine transplant on Feb. 24, 2016 as part of a clinical trial of the procedure. A vascular Candida infection caused complications that necessitated removal of the transplanted uterus 12 days after the surgery.
Following an extensive review and revision of the transplant program’s protocols, the Cleveland Clinic team resumed the trial of the deceased-donor procedure in 2017.
To date, the program has undertaken a total of five transplants. Three were successful engrafts: One of those resulted in last month’s birth, and two are pending embryo transfer after in vitro fertilization. The remaining two transplants ended with hysterectomies.
A total of 10 patients eventually will undergo transplantation during the trial, allowing Cleveland Clinic researchers to gather data intended to help refine and improve patient selection and the surgery, recovery, immunosuppression, in vitro fertilization and delivery processes.
Participation in the clinical trial requires that candidates between the ages of 21 and 45 be intensively screened and evaluated by a team that includes bioethicists, obstetricians, gynecologists, transplant surgeons, infectious disease specialists, psychiatrists and social workers. The process is meant to ensure that each woman is medically suitable, understands the potential consequences of the procedure, and has the personality, social support and other prerequisites for a successful outcome.
“Uterine transplant is a procedure that involves risks and is done to treat a non-life-threatening condition, unlike most transplants,” Dr. Tzakis says. “Patients are willing to take that risk because they want to have a baby of their own.”
Patients who are selected for the clinical trial must first undergo in vitro fertilization (IVF) and cryopreservation of at least six embryos harvested by a team of IVF specialists. That is followed by a wait for a matching deceased donor (a woman of reproductive age who has previously given birth), the transplant surgery, immunosuppression and infection prophylaxis, embryo transfer, caesarean delivery and eventual hysterectomy to remove the graft after one or two pregnancies.
The transplant itself entails a high degree of coordination, communication and surgical expertise across multiple specialties. In the procurement surgery, the dissection and preparation of the donor uterus is performed first, although the uterus remains in situ and perfused until cross-clamping and removal of lifesaving organs is completed by other transplant teams. Once obtained, the donor uterus is prepared for transplant by the procurement team while the uterus transplant team prepares the recipient in an adjacent operating room.
The transplant procedure involves suturing the uterine vessels — a pair of arteries and veins on each side of the organ — to the recipient’s vessels, after which the vaginal tissues are connected to the recipient’s gynecological anatomy. Proper orientation of the uterine vasculature is important to avoid kinking, which can cause thrombosis.
The team’s transplant surgeons are responsible for the vascular connections, while the gynecological surgeons are responsible for the gynecological connections and for ensuring that the graft is securely sewn into the pelvis to stabilize the uterus for future pregnancies. The procedure involves an area deep in the pelvis, dissection near vital structures and suturing of small vessels, which demands highly focused attention, skill and teamwork.
In this case, Drs. Tzakis and Falcone procured and prepared the donor organ. Transplant surgeons Cristiano Quintini, MD, and Koji Hashimoto, MD, PhD, performed the vascular anastomoses in the recipient. The gynecology team performed the vaginal anastomoses. Director of Transplantation Charles Miller, MD, provided critical oversight.
“Because of the complexity of the procedure, it’s not just about putting in a stitch and making a connection; it’s about geometry and strategy and orientation,” Dr. Quintini says. “Dr. Miller was supervising all the phases of the operation. It was like an orchestra. You need a conductor to make sure everything works smoothly and is coordinated. It was really beautiful. This was teamwork at the extreme.”
Embryo transfer was performed by the IVF team, led by obstetrician/gynecologist Rebecca Flyckt, MD, about six months after transplant. Conception was achieved after a single cycle.
Postoperatively, thrombosis and/or rejection of the graft can occur and must be closely monitored and managed. The patient underwent angiograms to confirm the patency of the transplant anastomoses, periodic cervical biopsies to monitor for rejection, as well as regular checks for pre-eclampsia, a potential complication from immunosuppressive agents.
The immunosuppressive regimen — involving azathioprine, prednisone and tacrolimus, and managed by the transplant team — is similar to that used following renal transplantation. “These medications are not anything new in terms of their usage during pregnancy,” Dr. Perni says. “There is extensive experience worldwide caring for women who are pregnant after kidney transplant or other transplants.”
Although the pregnancy was considered high-risk, Dr. Perni says it was notable for its relative normalcy. “The most remarkable thing was that it wasn’t remarkable. In terms of routine ultrasounds and prenatal care, everything was very similar to other pregnancies we manage.”
The collaborative care — from evaluation to IVF to transplant to embryo transfer to gestation and delivery — involved a multitude of specialists and a high degree of reliance on each other’s expertise.
“In general, when a transplant takes place, the transplant surgeon maintains a critical role for a long time,” Dr. Tzakis says. “In a uterine transplant, the control shifts to others, from IVF specialists to neonatologists. “It really takes a first-class team to work in harmony. It takes a lot of trust. This is one of the reasons I joined Cleveland Clinic.”
When it was time for the Caesarean delivery, the entire team was present. Dr. Perni led the procedure. The infant was born en caul — an unusual presentation in which the baby is still encased in the intact amniotic sac, which must be ruptured by the surgeon. Seeing the child appear was an emotional peak.
“We had all lost sleep trying to think of the possible complications and how to handle them,” Dr. Tzakis recalls. “Suddenly, the baby is there, and she is totally normal. The happiest day of my life was the birth of my daughter. This was the second-happiest day.”
“In other types of transplants, we quantify success by the patient going home and having a meaningful life,” Dr. Quintini says. “In this transplant, the result is a new life. It was an amazing moment.”
Dr. Perni praised the patient, the donor, and her colleagues.
“The patient was wonderful to work with, very cooperative,” she says. “She was willing to participate in this trial without any guarantee of success, with the intent of helping women in the future.
“Everyone is extremely grateful to the donor, without whose contribution this would not have been possible.
“And it was such a great honor and privilege to be a part of this amazing team, to work so closely with other specialists, all sharing the same goal,” Dr. Perni says.” It was truly an incredible experience.”
The clinical trial will continue.
“We have another two women with transplanted uteruses in place awaiting embryo transfer, and several other patients on our waiting list for transplant,” Dr. Tzakis says. “As we learn more, we will make adjustments to try to make the transplant safer and more efficient. The objective of the trial is to make transplantation an option, like surrogacy and adoption, for women with uterine factor infertility, so they will have more choices to have the babies they want.”

In rehabilitation medicine, the answer might require nuance

Mental health colleagues can provide much-needed perspective

Palliative care specialists know hard conversations can also be valuable ones

Variables affect nuances of the conversation
Authors discuss ethical challenges associated with sponsored genetic testing

Program focuses on nurturing ethics leaders in daily practice
Medical, ethical and legal considerations
An end-of-life dilemma in the intensive care unit