Expert insights and a thromboprophylaxis protocol
Severe COVID-19 is associated with a hypercoagulable state that may require different anticoagulation strategies and protocols to prevent and treat deep vein thrombosis (DVT) and pulmonary embolism (PE) in hospitalized patients. So noted Marcelo Gomes, MD, in a recent Tall Rounds online CME activity from Cleveland Clinic covering thrombotic complications and hypercoagulability associated with COVID-19.
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
“Hospitalized patients with severe COVID-19 appear to fall into a higher risk category for DVT/PE than one would expect from patients with other viral infections,” said Dr. Gomes, a staff vascular medicine specialist in Cleveland Clinic’s Department of Cardiovascular Medicine. “This means that a differentiated approach to prophylaxis and treatment may be necessary.”
Anticoagulation therapy alone is indicated for acute DVT/PE in hemodynamically stable patients, but patients with severe COVID-19 in the ICU may develop hemodynamic shock and refractory respiratory failure. When such high-risk patients develop a submassive or massive PE, consideration should be given to systemic thrombolysis or one of the following advanced strategies:
“Whether systemic therapy is more effective than catheter-directed therapies for patients with COVID-19, who often have multiple microemboli, is a timely and highly pertinent question for clinical research trials,” said Dr. Gomes.
A hemodynamically stable patient with COVID-19 who has an acute DVT/PE should be initially treated with one of the following, Dr. Gomes noted:
In most patients with acute DVT/PE, the activated partial thromboplastin time (aPTT) is considered adequate for monitoring IV UFH (if baseline aPTT is normal). But in COVID-19, its reliability is called into question for a number of reasons, including the fact that aPTT is often prolonged in patients with severe disease and the reality that many others will develop coagulopathy during the course of their illness.
Furthermore, heparin resistance often develops because of increased levels of fibrinogen, factor VIII or von Willebrand factor, leading to difficulty achieving therapeutic levels despite multiple incremental doses of heparin. In addition, lupus anticoagulants have been commonly reported in hospitalized COVID-19 patients, leading to a prolonged baseline aPTT, which makes it unreliable for monitoring IV UFH therapy.
Because of the multiple issues with aPTT monitoring, Dr. Gomes stated, the anti-Xa assay is the preferred method for monitoring IV UFH in patients with severe COVID-19.
Direct oral anticoagulants (DOACs) should be avoided in hospitalized patients with COVID-19, Dr. Gomes warned. “That’s especially the case in those receiving experimental COVID-19 treatments and in those with acute kidney injury, which is quite common in severe cases,” he said. Many patients require hemodialysis, which is a contraindication to DOACs.
Moreover, Dr. Gomes added, experimental anti-viral therapies for COVID-19 have the potential for strong drug-drug interaction with DOACs, dangerously reducing or raising plasma levels of the DOAC, thereby increasing the risk of either thrombosis or bleeding. A study from Northern Italy showed exponentially increased plasma levels of DOACs in such patients. Notably, the University of Liverpool has posted a comprehensive online tool for drug interactions that includes experimental COVID-19 drugs and indicates whether specific drug combinations are contraindicated.
“Interactions are not problematic between parenteral drugs and the experimental therapies,” Dr. Gomes noted. “And once patients are discharged and not on experimental COVID-19 therapies, DOACs can likely be resumed safely.”
Patients with severe COVID-19 in the ICU appear to have a peculiar hypercoagulable state, which predominantly manifests with one of the following:
Interestingly, Dr. Gomes noted, “preliminary evidence seems to indicate that the incidence of venous thromboembolism in mild to moderate COVID-19 — that is, in hospitalized patients not in the ICU — doesn’t appear to be higher than in patients hospitalized for other medical conditions, although the true incidence is still unknown.”
D-dimer and prothrombin time have been documented to correlate with a worse prognosis in patients with COVID-19. A D-dimer level twice the upper limit of normal (ULN = 500 ng/mL FEU) predicts higher mortality, disease progression, development of acute respiratory distress syndrome and need for ICU care. Higher D-dimer (five to six times the ULN) is also associated with higher risk of acute DVT/PE.
Dr. Gomes shared Cleveland Clinic’s protocol for thromboprophylaxis in hospitalized patients with COVID-19 (see figure below), which is based on a consensus among a multidisciplinary group of specialists in vascular medicine, hematology, intensive care medicine, hospital medicine and pharmacy. Risk of DVT/PE is stratified based on D-dimer levels, with six times the ULN (3,000 ng/mL FEU) chosen as the threshold for intensifying thromboprophylaxis from standard to a higher (“intermediate”) dose of enoxaparin. Recommendations for post-discharge thromboprophylaxis vary depending on risk stratification (see figure). High-risk patients may be prescribed enoxaparin or a DOAC for five weeks.
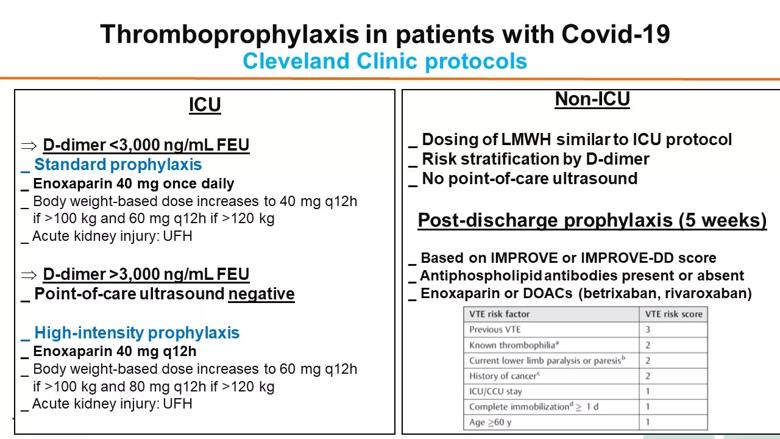
“The best thromboprophylactic strategy is a matter of fierce debate,” Dr. Gomes said. “Until results of randomized trials comparing strategies are available, recommendations must be based on preliminary retrospective evidence, and we need to maintain flexibility as more evidence becomes available.”
A number of principles from Dr. Gomes’ Tall Rounds talk were echoed in a case study that launched the Tall Rounds session, presented by session moderator Deborah Hornacek, MD.
A 54-year-old man was transported to Cleveland Clinic by EMS in April 2020 following a presyncopal event with an unwitnessed fall at home that had been preceded by more than a week of fever, chills and myalgia. His past medical history included hypertension and type 2 diabetes. He was found to be hypoxic and hypotensive by EMS. His blood pressure improved after fluid resuscitation, and lab testing was remarkable for a D-dimer level of nearly 18,000 ng/mL. After an initial negative result, repeat COVID-19 testing was positive. CT showed a massive saddle PE, and Cleveland Clinic’s multidisciplinary pulmonary embolism response team (PERT) was convened to review the case.
“Given the patient’s presentation with persistently low blood pressure and increasing cardiac biomarkers in the setting of severe COVID-19 pneumonia, we deemed him a candidate for advanced therapy,” noted Dr. Hornacek, a staff physician in the Section of Vascular Medicine. His recent unwitnessed fall raised concern about using thrombolytic therapy, so the team decided to proceed with bilateral pulmonary artery aspiration thrombectomy, which was successful.
The patient’s hemodynamics improved immediately after aspiration, and he continued to fare well afterward. Notably, his aPTT did not correlate with heparin levels, so anti-Xa assays were used to monitor IV UFH in the hospital. He was discharged home on the DOAC apixaban on hospital day 7.

Patients report improved sense of smell and taste

Clinicians who are accustomed to uncertainty can do well by patients
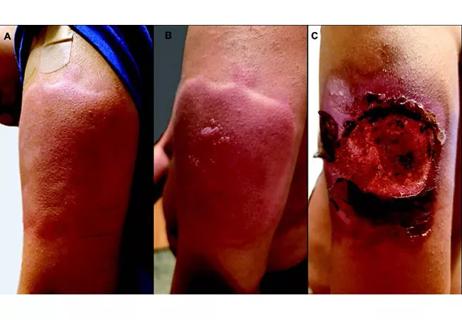
Unique skin changes can occur after infection or vaccine

Cleveland Clinic analysis suggests that obtaining care for the virus might reveal a previously undiagnosed condition
As the pandemic evolves, rheumatologists must continue to be mindful of most vulnerable patients

Early results suggest positive outcomes from COVID-19 PrEP treatment

Could the virus have caused the condition or triggered previously undiagnosed disease?
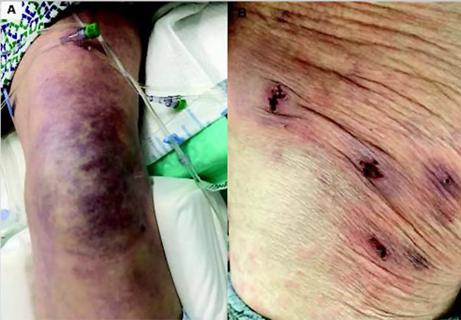
Five categories of cutaneous abnormalities are associated with COVID-19